Zika Vaccines
Zika Virus Vaccine Candidates 2024
Research on Zika virus (ZIKV) vaccine candidates has been active in 2024. Vaccination is considered the best strategy for the prevention of ZIKV-induced clinical issues. Developing a safe and efficacious Zika vaccine is a global health priority, says the World Health Organization (WHO). As of March 11, 2024, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have not approved any Zika vaccine or treatment. Clinical trials involving DNA, modified vaccinia Ankara vector platform, and purified inactivated vaccine candidates (Jan. 2023, May 2023, Oct. 2023) show that vaccines can induce neutralizing antibodies. Since 2016, approximately $350 million of research funding for a Zika vaccine has been mobilized (Chapman et al. 2020, U.K.'s Newton Fund, U.S. HHS). The US NIAID is developing multiple vaccine candidates to prevent Zika virus infection.
VLA1601 is a highly purified, inactivated, adjuvanted Zika vaccine candidate adsorbed on aluminum hydroxide. On March 26, 2024, Valneva announced initiating an additional Phase 1 clinical trial (VLA1601-102) of VLA1601, its second-generation adjuvanted inactivated vaccine candidate against the ZIKV.
ZPIV is a Zika virus vaccine candidate with purified formalin-inactivated Zika virus. In June 2023, a study found ZPIV to be well tolerated in flavivirus-naive and primed adults; bat immunogenicity varied significantly according to antecedent flavivirus vaccination status. Immune bias towards the flavivirus antigen of initial exposure and the timing of vaccination may have impacted responses. In this phase 1 clinical trial, a third ZPIV dose overcame much, but not all, of the discrepancy in immunogenicity.
TAK-426 (PIZV) is a purified, inactivated, alum-adjuvanted, whole Zika virus vaccine candidate. It is being tested to provide safety and immunogenicity data for further clinical development.
iosBio OraPro-Zika is an orally administered Zika virus vaccine candidate based on a non-replicating human adenovirus type 5 (AdHu5) (E1/E3 deleted) expressing Zika transgenes.
GEO-ZM02 is constructed using a modified vaccinia Ankara vector platform. Preclinical studies demonstrated that a single dose of GEO-ZM02 provided 100% protection against a lethal dose of the Zika virus. This Zika vaccine is based on the virus's NS1 protein ka, which is not associated with antibody-dependent enhancement of infection.
Ad26.ZIKV.001 is a replication-incompetent human adenovirus serotype 26 (ad26) vector vaccine candidate. In a clinical trial, researchers found two doses of oAd26. ZIKV. 001 were safe, causing mild to moderate reactogenicity and induced persistent neutralizing antibody responses. The single dose had lower peak antibodies but was durable after one year.
GeneOne Life Science and Inovio Pharmaceuticals DNA vaccine GLS-5700's phase 1, open-label clinical trial elicited anti-ZIKV immune responses.
VRC5283 is a Zika virus DNA vaccine candidate composed of a single closed-circular DNA plasmid encoded with wild-type precursor transmembrane M and envelope proteins from the H/PF/2013 strain of ZIKV. It is being tested in a phase 2 clinical study.
The University of Liverpool researchers at the Clinical Research Facility within the Royal Liverpool University Hospital said that on April 27, 2023, participants would receive two doses of the new vaccine. Up to 40 volunteers are planned for this phase of work, which will take place over the next nine months.
The University of Adelaide was awarded $1.35 million in funding to develop a novel DNA vaccine (pVAX-tpaNS1) for the Zika virus. Dry-coating of pVAX-tpaNS1 on the HD-MAP device resulted in no loss of vaccine stability at 40°C storage over 28 days.
rZIKV/D4Δ30-713 is a live attenuated chimeric Zika candidate vaccine expressing the premembrane (prM) and envelope (E) genes of a contemporary ZIKV strain within a dengue DEN4Δ30 background. It completed a phase 1 clinical trial. The researchers wrote - Our results suggest rZIKV/DEN4Δ30 is over-attenuated and thus will not be further developed as a candidate ZIKV vaccine.
ZikaEnv:aghFc is a plant-based recombinant vaccine that transiently expresses the ZIKV envelope protein. At a low dose (1–5 μg), it induces both humoral and cellular immunity.
Duke-NUS researchers used Zika virus live-attenuated vaccine (ZIKV-LAV) strains, which are "weakened" viruses with limited ability to infect healthy cells.
Zika Outbreaks
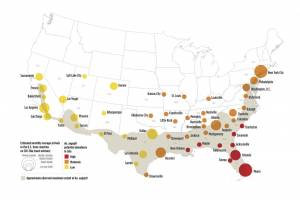
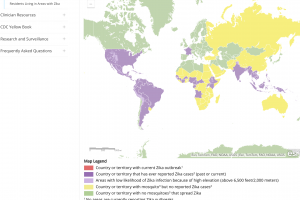
Zika outbreaks have been confirmed in numerous countries in 2024.
Zika Vaccine News
December 27, 2023 - A study showed that our plant-based recombinant subunit ZIKV vaccine afforded substantial protective immunity. This immunity was vertically transferred, protecting the next generation of mice against ZIKV infection.
December 2, 2023 - A needle-free vaccine patch is being developed to protect people from the Zika virus. A prototype high-density microarray patch shows promise in preclinical studies for delivering a novel, needle-free vaccine against the Zika virus.
October 21, 2023 - Scientists deliberately infected people with Zika virus to learn whether such a strategy could help to test vaccine candidates.
June 27, 2023 - The Lancet Infectious Diseases published a phase 1 study that found ZPIV was well tolerated in flavivirus-naive and primed adults, and humoral immune responses rose substantially following a third dose of ZPIV.
May 2, 2023 - The first participant has received a dose of a new Zika virus vaccine being trialed by the University of Liverpool at the Clinical Research Facility within the Royal Liverpool University Hospital, based on work at the University of Manchester.
April 19, 2023 - Article: Scientists find different flavors of broadly neutralizing antibodies that could lead to new vaccines for growing threats.
April 1, 2023 - The journal Nature published results from a study: An effective live-attenuated Zika vaccine candidate with a modified five ′ untranslated region. These results suggest that modifying the ZIKV 5′ UTR is a novel strategy to develop live-attenuated vaccine candidates for ZIKV and potentially for other flaviviruses.
March 30, 2023 - Zika vaccines will be discussed at the 23rd World Vaccine Congress in Washington, D.C., on April 4, 2023.
February 21, 2023 - Medcape reported: Zika Virus Still Calls for Preparedness and Vaccine Development.
January 25, 2023 - GeoVax Labs, Inc. confirmed the U.S. Patent and Trademark Office issued a Notice of Allowance for Patent Application No. 17/000,768 titled, "Method for Generating a ZIKV Immune Response Utilizing a Recombinant Modified Vaccinia Ankara Vector Encoding the NS1 Protein."
January 19, 2023 - The Lancet Infectious Disease published a study: The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomized, placebo-controlled, dose-ranging, phase 1 clinical trials. These findings support the continued development of mRNA-1893 against the ZIKV.
May 18, 2021 - The Lancet published a study that concluded that TAK-426 was well tolerated, had an acceptable safety profile, and was immunogenic in both flavivirus-naive and flavivirus-primed adults. Based on the safety and immunogenicity profiles of all TAK-426 doses assessed, the 10 μg TAK-426 amount was selected for further clinical development.
February 16, 2021 - The Zika virus candidate, Ad26.ZIKV.001, a replication-incompetent human adenovirus serotype 26 (ad26) vector, showed promising safety and immunogenicity in phase I clinical trials. Researchers say the vaccine warrants further development should the need reemerge.
August 7, 2020 - A new study describes the preclinical selection and development of a potent ZIKV vaccine from 9 constructs using SAM technology expressing various forms of the ZIKV prM-E antigen. This study identified one ZIKV SAM vaccine candidate, VRC5283, and two wholly protected mice and nonhuman primates from the ZIKV challenge.
April 15, 2020 - Moderna's Zika vaccine mRNA1893 low dose levels were very effective.
December 19, 2019 - A U.S. NIH-developed Zika vaccine improves fetal outcomes in an animal model.
November 28, 2017 - Takeda Pharmaceutical announced that its purified, inactivated, alum-adjuvanted whole Zika virus vaccine candidate (TAK-426) has progressed into a Phase 1 clinical trial.
Note: Zika vaccine content sources include, but are not limited to, the World Health Organization, the US Centers for Disease Control and Prevention, clinicaltrials.gov, and the Precision Vaccinations news network. Healthcare providers have reviewed this content.